Understanding the electrosorption of confined charged species
Batteries and electric double-layer capacitors (EDLCs) are electrochemical energy storage devices that are differentiated by their individual charge storage mechanism. They are both mainly composed of two electrodes and an ion-containing electrolyte, however, the mechanism in which ions and electrodes interact is distinctly different.
In a battery, during insertion, the electrolyte ions are separated from their solvation envelope before being inserted into the electrode material, where they undergo so-called redox reactions in which charges are transferred between electrode and ions. Contrarily, in an EDLC, solvated ions are adsorbed at the surface of the electrode and form an electric double-layer, in which charges are separated between electrode and ions. These differences mean that the batteries can storage large amounts of energy, while taking long times to charge and discharge, whereas it is the other way around in the EDLC.
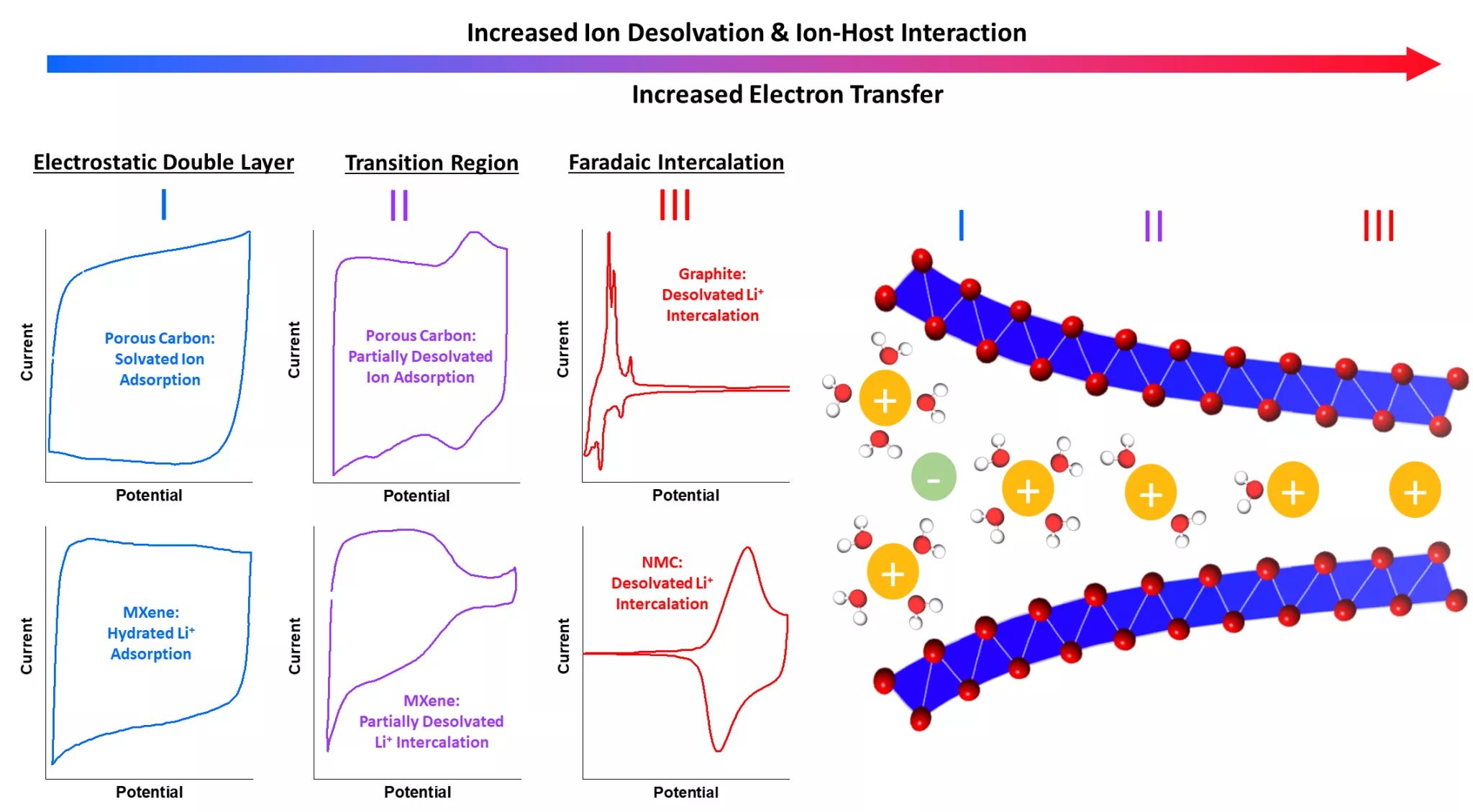
The two charge storage mechanisms have been strictly separated for decades, but in a recent publication in “Nature Energy”, researchers now suggest that there can be a continuous transition between them. In porous and layered electrode materials, ions can be adsorbed while only being partially solvated, depending on the size and geometry of the pore or the interlayer space. The researchers suggest that in this so-called nanoconfinement region, charge storage processes can lie on a spectrum between classic electric double-layer formation and battery-like redox reactions, depending on the level of ion solvation. In this nanoconfinement region, so-called pseudocapacitive charge storage processes can be observed.
Bridging the divide between classic batteries and EDLCs offers the opportunity to combine the “best out of both worlds”: Electrochemical energy storage devices with a combined high energy and power.
Read the entire article here.

Simon Fleischmann is a principal investigator at the Helmholtz Institute Ulm & Karlsruhe Institute of Technology in Germany, where he started an independent research group funded by the German Federal Ministry of Education and Research.
Simon first studied Materials Science and Engineering at Saarland University in Saarbrücken, Germany, and Luleå University of Technology, Sweden, and obtained his M.Sc. degree in 2016. He completed a dissertation at INM – Leibniz Institute for New Materials and Saarland University in the group of Prof. Volker Presser where he investigated hybrid electrochemical energy storage materials and devices. He obtained his Ph.D. in 2018. Afterwards, he spent nearly two years as a postdoc in the group of Prof. Veronica Augustyn at North Carolina State University in the U.S., where his research focused on the synthesis and the charge storage mechanism in interlayer-functionalized materials.
Simon joined the RS2E in the beginning of 2021 in the group of Prof. Patrice Simon at Université Paul Sabatier & CIRIMAT as part of the RS2E Young Energy Storage Scientist Award (YESS AWARD). His project there consisted of the investigation of the charge storage mechanism in a layered model electrode material, titanium disulfide, via in situ diffraction and electrochemical impedance spectroscopy methods.
“My experience with RS2E was fantastic, both on a scientific and personal level. I was able to learn a lot and develop new skills under the supervision of Prof. Simon, especially in the area of electrochemical impedance spectroscopy. Besides, I really enjoyed the friendly atmosphere at the research institute and the beautiful city of Toulouse.”