A new strategy to overcome the limitations of anionic redox
“Promises but still a lot of roadblocks to break » could sum up the current state of knowledge on the anionic redox phenomenon. The promises to start with are those of high-performance Li-ion batteries, with a better energy density and increased capacity. The main drawbacks are a significant voltage fade, a large voltage hysteresis (voltage difference between charge/discharge), which means that some of the energy is lost through heat dissipation, and slow kinetics.
These limitations currently hinder the development of a viable commercial solution based on this phenomenon.
An international team led by Prof. Jean-Marie Tarascon, RS2E director, had the idea of subjecting a so-called "Li-rich" electrode material to harsh oxidation and reduction conditions to better understand its changes in structure and composition. Their results were published in the journal Nature Communications.
Harsh treatment
For their study, the scientist worked with an archetypical Li-rich material, Li1.2Ni0.13Mn0.54Co0.13O2, and subjected it to extreme oxidation (to 4,8V) and reduction (to 1,2V) conditions to monitor and characterize its evolution. The operating voltages of a battery are generally between 4,5V and 1V.
First observation, by oxidizing at 4,8V for a given period, the researchers identified in the studied material a new phase which had never been isolated before and whose structure has been found. Contrary to the previous beliefs, this new phase is not only on the surface but also in all the material. The oxidation time must be long enough to let the Li ions be extracted from the material and to allow the transition metals, in particular Mn, migrating in the inter-sheet space.
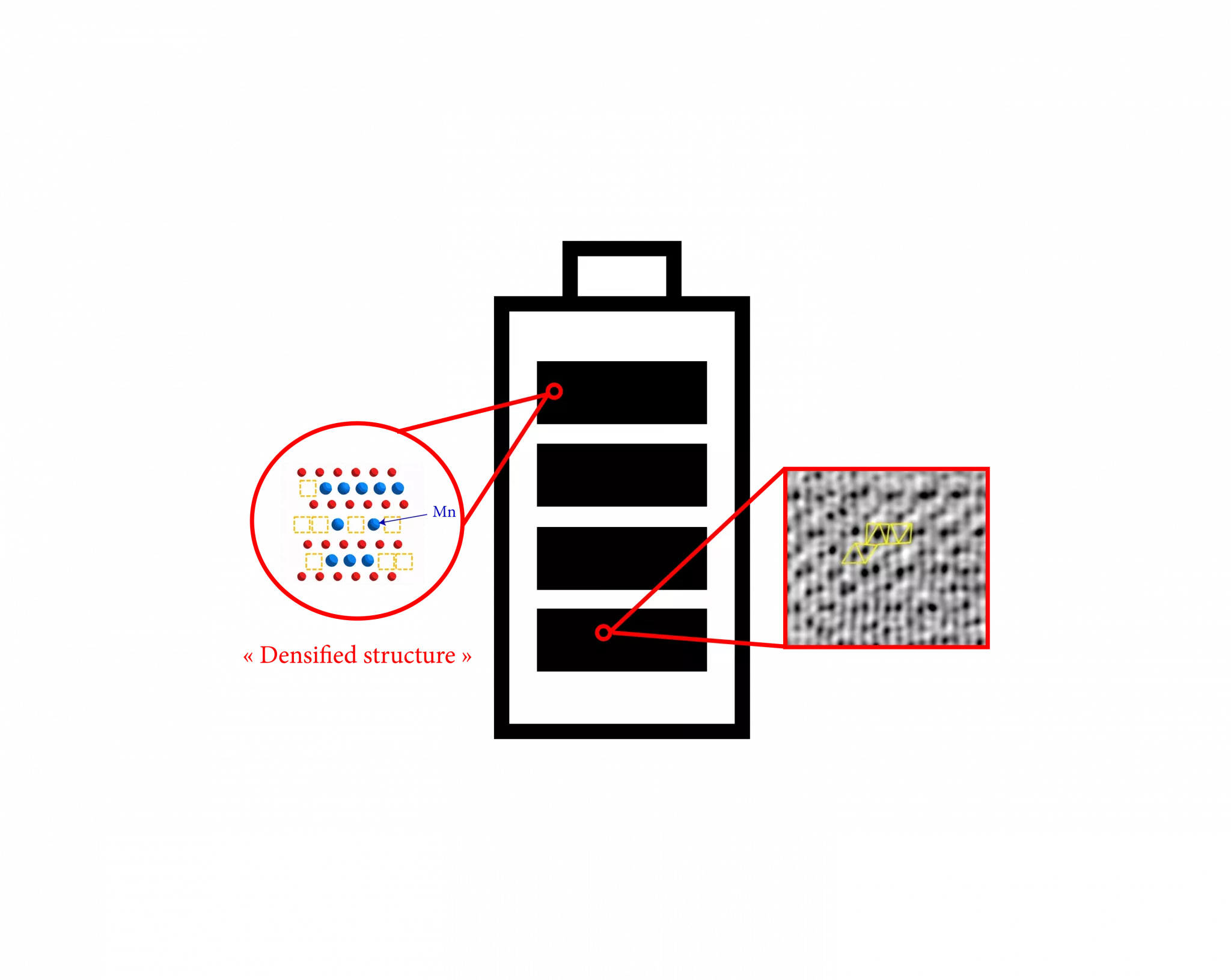
Scheme 1: Structure of the densified phase after a harsh oxidation
The appearance of a new phase is of paramount importance because at the times of discharge/reduction at 1,2V it is able to reinsert more Li ions than the “classic” Li-rich phases. Thus, it can compensate the loss of irreversible capacity observed in the materials tested for anionic redox.
Otherwise, in practice, the use of batteries integrating those materials at the mentioned extreme tensions damages the electrolyte, what affects the lifetime of Li1.2Ni0.13Mn0.54Co0.13O2 electrode. The fact remains that this extreme oxidation / reduction strategy could be applied in the future to other Li-rich materials.
References:
Structural evolution at the oxidative and reductive limits in the first electrochemical cycle of Li1.2Ni0.13Mn0.54Co0.13O2
Wei Yin et al.
Nature Communication, 06/03/20, DOI : 10.1038/s41467-020-14927-4
Contact : Jean-Marie Tarascon, jean-marie.tarascon@college-de-france.fr