MXene/electrolyte, the strategic duo to improve pseudocapacitors
An international team of researchers, whose some members of RS2E, demonstrates how simply changing the solvent of an pseudocapacitive system can drastically impact the performances of a Mxene Ti3C2 electrode in non-aqueous medium.
Batteries and supercapacitors are two energy storage systems with complementary properties: The first ones offer high energy densities while the second ones can be charged and discharged quickly. Batteries are the ideal systems for autonomy applications and supercapacitors for power applications.
Today, an important challenge is to increase the energy density of supercapacitors to enlarge their use field. To obtain that, scientists replace carbon electrodes in supercapacitors by pseucapacitive materials. In this case, the charge storage is done by fast surface-confined redox reactions. In batteries, charge and discharge are done by insertion/disinsertion of charge-storing ions in the electrodes.
The first studies in the field of pseudocapacitive materials were focused on the oxides (RuO2 and MnO2), but 2D metallic carbides (MXene) were quickly identified as promising new materials of electrodes. You can find all these conclusions in an article published in Nature Energy and explained in our website (in French).
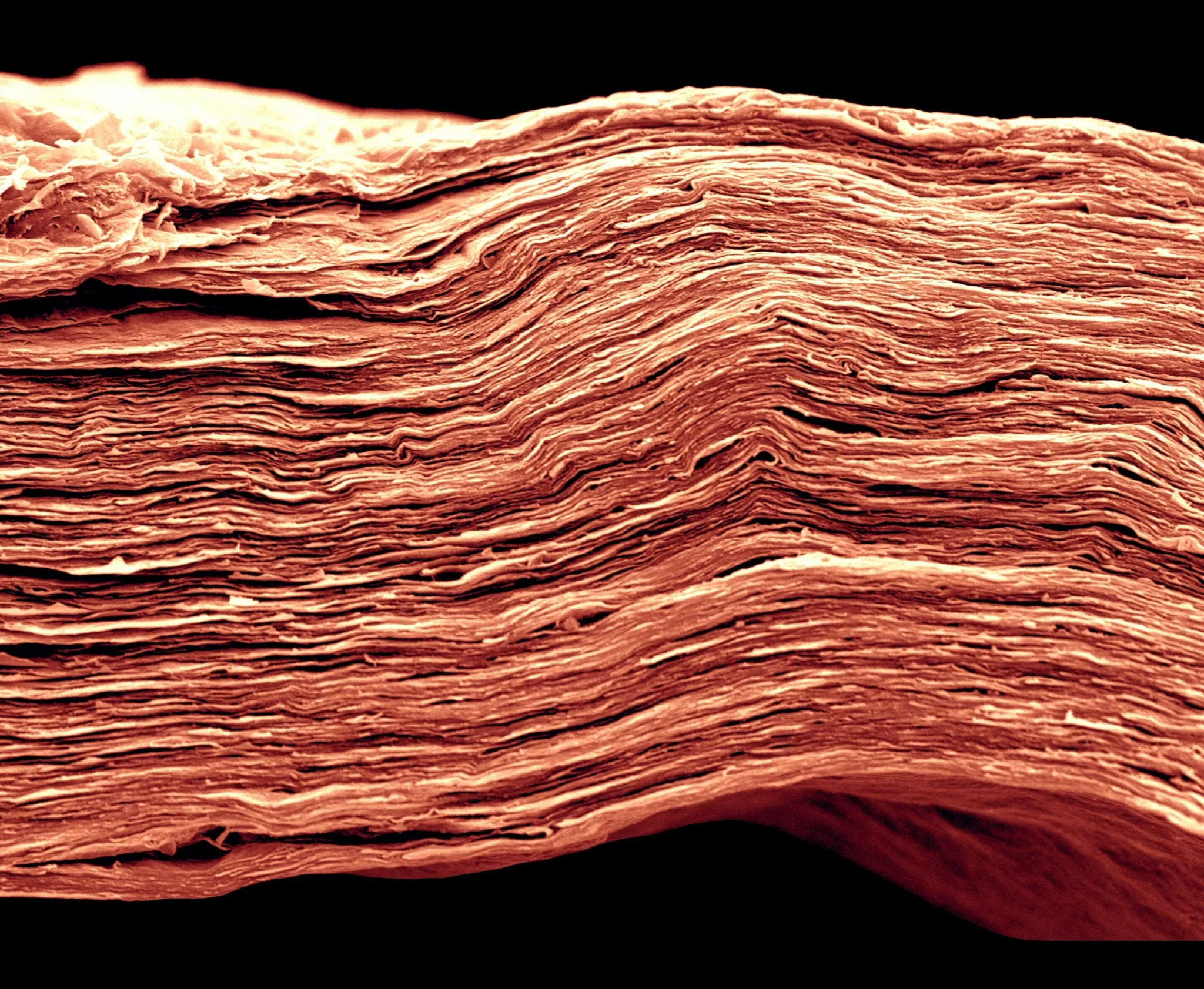
Picture 1 : A MXene film only 5 microns thick, SEM
Change of perspective
A new team, made up of former members from the previous Nature Energy article, demonstrates how simply changing the electrolyte of the pseudocapacitive system improves the performances of the MXene Ti3C2 electrodes. Moving from the study of the only electrode to the study of the electrolyte, the scientists highlight in a new Nature Energy paper the relevance of studying all the aspects of an electrochemical system to contribute to its development.
The challenge for the MXenes was to reproduce their outstanding performances by combining them with non-aqueous electrolytes, which offer cell voltages higher than those of aqueous electrolyte, and therefore higher energy densities.
Researchers have studied three different combinations of organic electrolyte using LiTFSI salts mixed with propylene carbonate (PC) or dimethyl sulfoxide (DMSO) or acetonitrile (ACN).
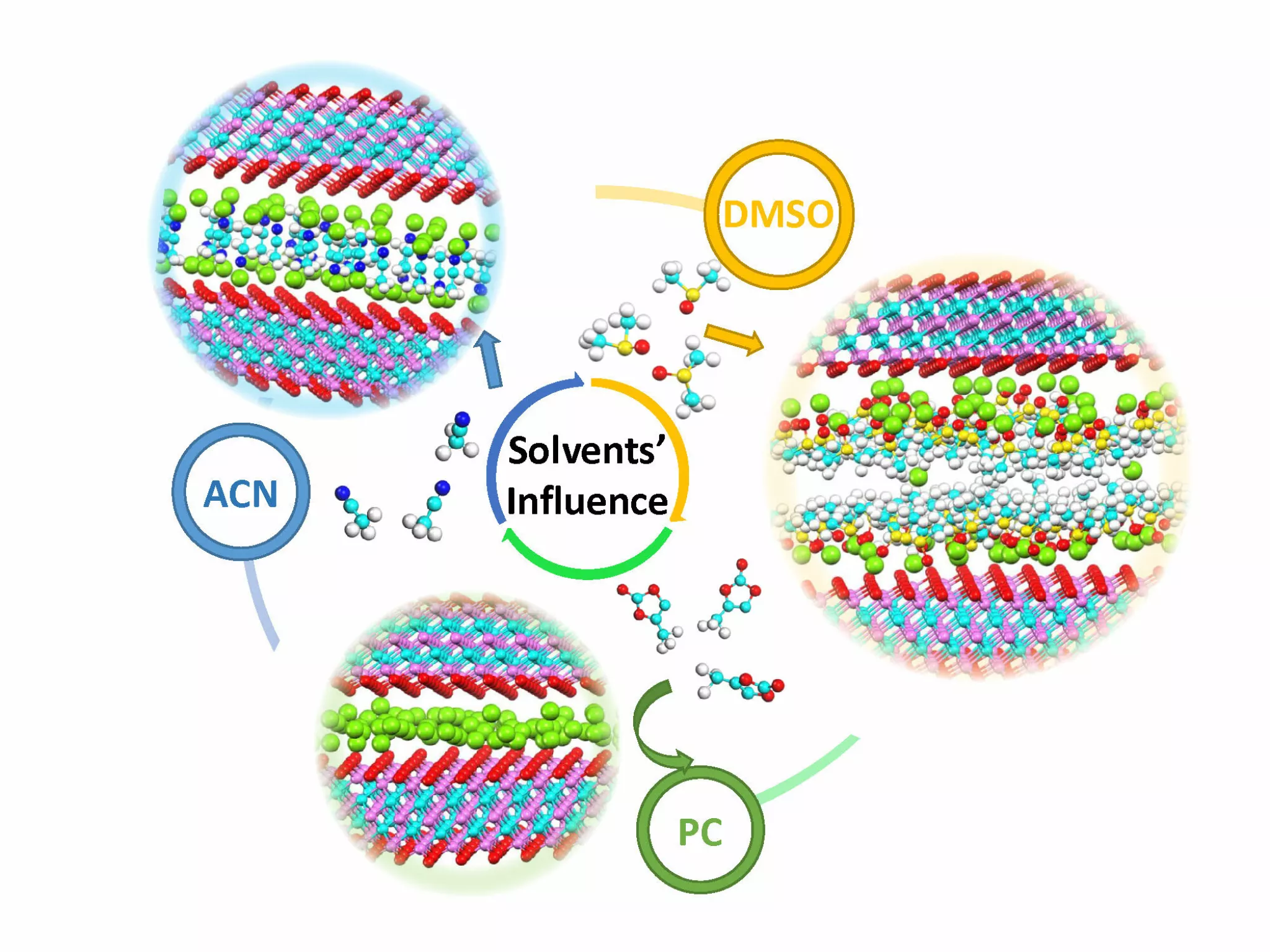
Figure 1: Influence of different solvents on the pseudocapacitive insertion of Li ions
Against all odds, ACN is not the best electrolyte. If with its high ionic conductivity, ACN would be the best choice for conventional supercapacitors, PC should be chosen for MXene Ti3C2 based pseudocapacitors.
The reason: PC allows complete desolvatation of the Li ions in the electrolyte and improves their pseudocapacitive intercalation in the MXene electrode.
The double “electrode/electrolyte” study is a winning bet et opens the way to a new way of designing complete and efficient pseudocapative systems.
References :
Influences from solvents on charge storage in titanium carbide MXenes
Xuehang Wang, Tyler S. Mathis, Ke Li, Zifeng Lin, Lukas Vlcek, Takeshi Torita, Naresh C. Osti, Christine Hatter, Patrick Urbankowski, Asia Sarycheva, Madhusudan Tyagi, Eugene Mamontov, Patrice Simon, Yury Gogotsi
Nature Energy, 04/03/2019, DOI : 10.1038/s41560-019-0339-9
Contact : Yury Gogotsi, gogotsi@drexel.edu