Lamellar catalyst and alkaline electrolyte, the winning combination
Energy storage is a scientific challenge that is becoming more and more urgent to solve. One of the solution envisaged by the scientists is to store energy from renewable sources in the form of a chemical vector with high industrial impact, hydrogen, using an electrolyser.
Unfortunately, electrolyser performance is limited by the slow kinetics of one of the reactions that occurs at the electrodes: The water oxidation and oxygen release reaction. To solve this problem, researchers are devising and studing different types of catalysts which, for the time being, remain too unstable when used.
An international team, bringing together several RS2E laboratories, demonstrates in an article published in Nature Communications that it is possible to couple a solid lamellar catalyst to an alkaline electrolyte to avoid destabilization.
Counterbalance the charges
Their work demonstrates that the lamellar structure of the studied electrocatalyst, α-Li2IrO3, allows the alkaline potassium-based electrolyte to be inserted inside the material when it reacts chemically with water (oxidation). Inserted K+ cations participate in the overall charge balancing. The catalyst can act without being destabilized and without its performance being affected. The cation insertion also allows the appearance of a new “birnessite” phase in the material, whose water oxidation activity is 5 times greater to normal phases.
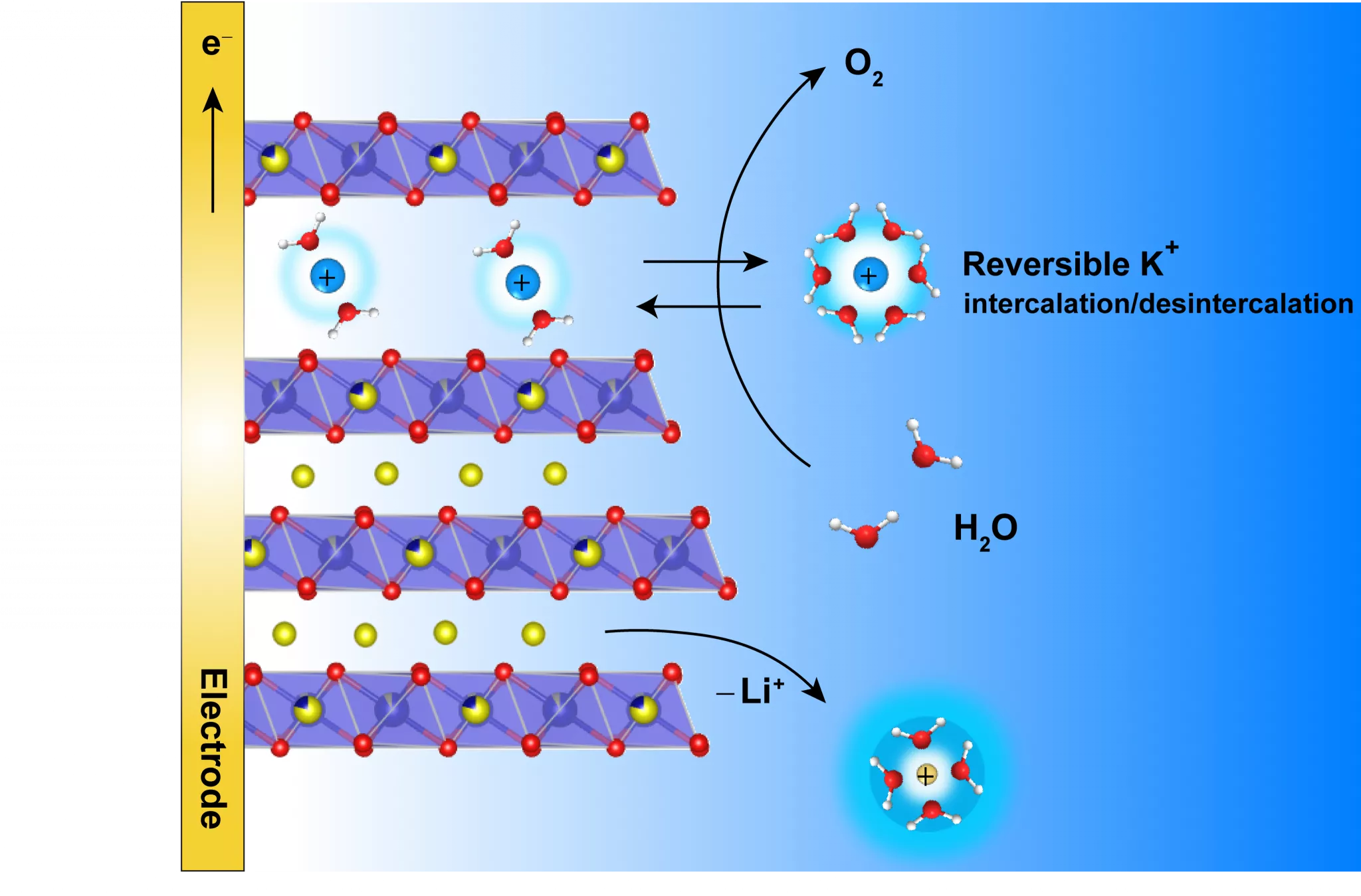
Scheme 1: K+ ion insertion to counterbalance charge balancing
Researchers still have to test this strategy on the scale of a true electrolyser to be sure of its viability. Nonetheless, this strategy is already an interesting line of thought for the design of future catalysts via the coupled optimization of a catalyst and the support electrolyte. The researcher team demonstrated that its observations can be applied to another lamellar material, LiCoO2, which has the advantage of not containing iridium, a rare transition metal.
References:
Cation insertion to break the activity/stability relationship for highly active oxygen evolution reaction catalyst
Chunzhen Yang et al.
Nature Communication, 13/03/30, DOI : 10.1038/s41467-020-15231-x
Contact : Alexis Grimaud, alexis.grimaud@college-de-france.fr